
Very few people know their HIV status and others have never even been to a VCT center. The number of people contracting HIV has been on the rise over the past few years and the statistics especially in Africa have been alarming.
While the scientists are yet to discover a cure for the HIV virus, six countries including Kenya, Malawi, Botswana, Zimbabwe, South Africa and Swaziland will be part of a large scale trial for the antiretroviral (ARV) drug for Pre-Exposure Prophylaxis (PrEP).
Uganda has started recruiting women for trials of the long-lasting injectable ARV drug and it is expected that a total of 3,200 individuals from across the seven countries will also be recruited for the study that will run for over a five-year period.
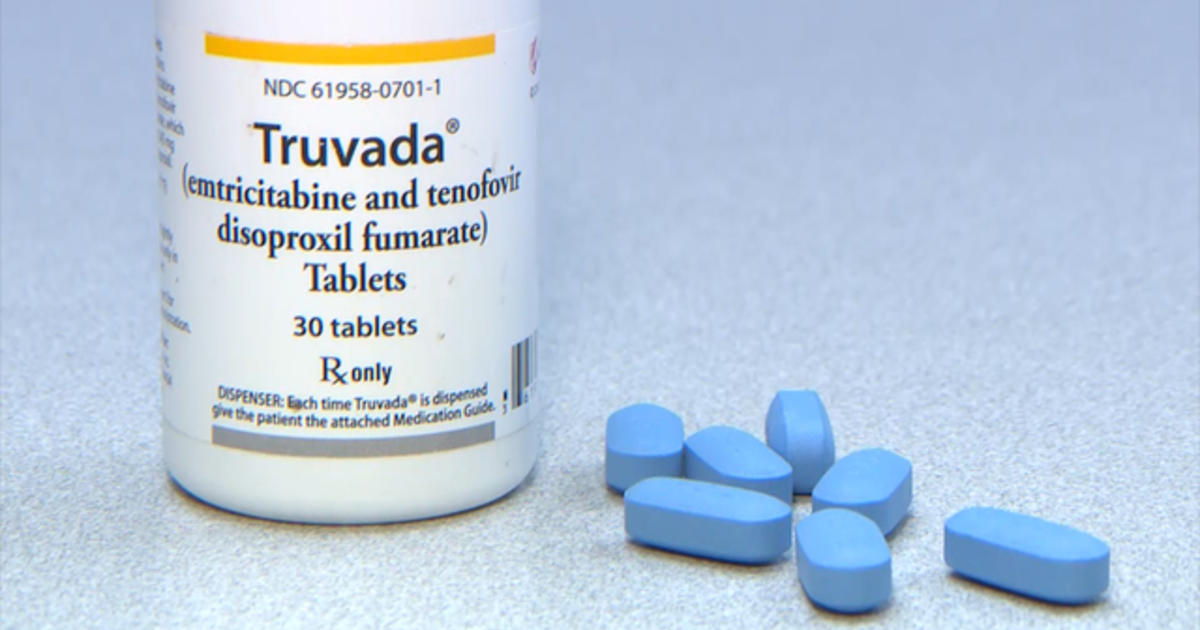
Known as HPTN084, the phase III clinical trial aims to assess if an injectable ARV called Cabotegravir, administered every two months is safe and can reduce an individual’s risk of acquiring HIV when they are exposed. If the drug turns out to be safe it could become the drug of choice for use as PrEP, instead of the current Truvada, an oral pill that must be taken daily.
Truvada, an oral pill, is the drug being used by HIV negative individuals to reduce their risk of infection in case of exposure to HIV. Because the drug must be taken daily, health experts say it has seen in poor adherence in many cases.
Dr. Amanda Wanyana, a researcher with the International Aids Vaccine Initiatives, at the Uganda Virus Research Institute, one of the sites where the trial will be conducted, noted that while in Southern Africa it will be carried out among HIV-negative with focus on high risk groups including gays, in countries such as Uganda and Kenya, the trial will focus on HIV negative, healthy women of reproductive age, 18 to 45 drawn mainly from fishing communities.
The trial will be blinded, meaning participants will be divided into two groups. One group will be given the injection with the active ingredient under study, while the other will be given a placebo.
According to Dr. Wanyana, trial participants will receive the injection once every month for the first two months, and thereafter, they will receive one injection every two months for the rest of the study period.

Women who take part in the study will also be expected to be on long term contraception, be in good clinical health and not be pregnant at the time of recruitment or during the trial.
“Besides issues of efficacy, we also want to know how the drug affects reproduction in women, and if there are cases of drug resistance. In the long run, if the injectable ARV works as well, it will be an option as PrEP,” said Dr. Wanyana.
“A long-lasting injectable PrEP, which can be administered every two months, can therefore help people who are unable to adhere to daily oral pills,” said Dr. Wanyana.

Currently, the drug is being administered to high-risk individuals such as sex workers and couples in discordant relationship through a pilot programme in selected health facilities.
Gertrude Nanyonjo, a community engagement officer said sensitisation programmes are currently being undertaken in communities where the trials will be conducted to raise awareness among women about the benefits of taking part in the study.
“We emphasise the fact that women are more vulnerable to acquiring HIV and how they will benefit from the research,” said Ms Nanyonjo.
Did you know that according to estimates from the Joint United Nations Programme on HIV/Aids, Girls and women make up more than half of the 36.7 million people currently living with the virus?